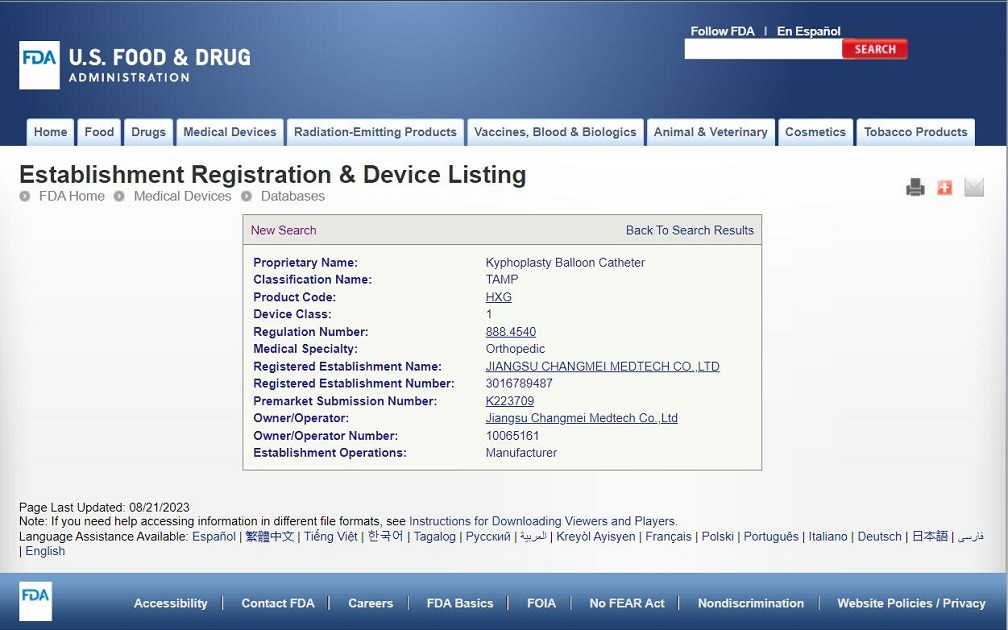
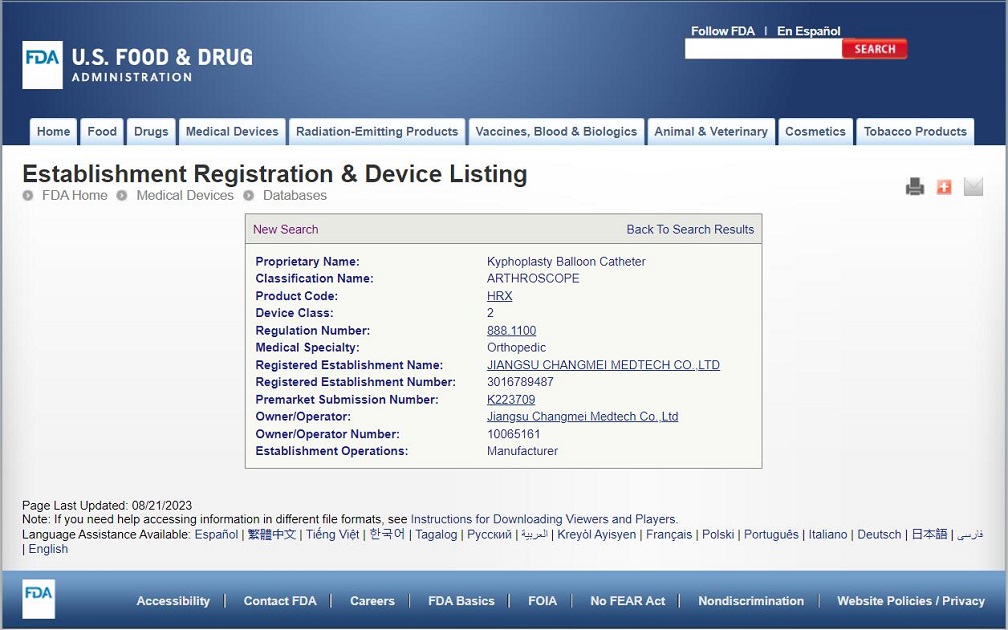
Congratulations to CHANGMEI's kyphoplasty balloon catheter for obtaining FDA certification
Congratulations to CHANGMEI's kyphoplasty balloon catheter for obtaining FDA certification, K223709. MDR is also in the process of conversion and is expected to obtain MDR certification in 2024.





 CopyRight © 2024 - Jiangsu Changmei Medtech Co., Ltd.All rights reserved
CopyRight © 2024 - Jiangsu Changmei Medtech Co., Ltd.All rights reserved
