Landmark Success: Obtaining EU MDR CE Certificate
Jiangsu Changmei Medtech Co., Ltd.'s achievement in obtaining the EU MDR CE certificate for its Kyphoplasty System Products(kyphoplasty balloon catheter, kyphoplasty tool kit, and balloon inflator) represents a watershed moment in the company's journey toward global recognition and success.
Overcoming the challenges and complexities associated with regulatory compliance, Changmei Medtech has demonstrated its resilience, ingenuity, and commitment to excellence.
As the first Chinese company to receive EU MDR certification for Kyphoplasty System Products, Changmei Medtech has paved the way for other domestic manufacturers to follow suit and establish a strong presence in the global market. This milestone not only highlights the company's achievements but also reflects the transformative potential of Chinese medical technology on the global stage.
Looking ahead, Jiangsu Changmei Medtech Co., Ltd. is poised to capitalize on its EU MDR certification to drive further innovation, expansion, and impact in the medical device industry. With a steadfast dedication to quality, compliance, and customer satisfaction, Changmei Medtech is well-positioned to shape the future of healthcare and improve the lives of patients worldwide.
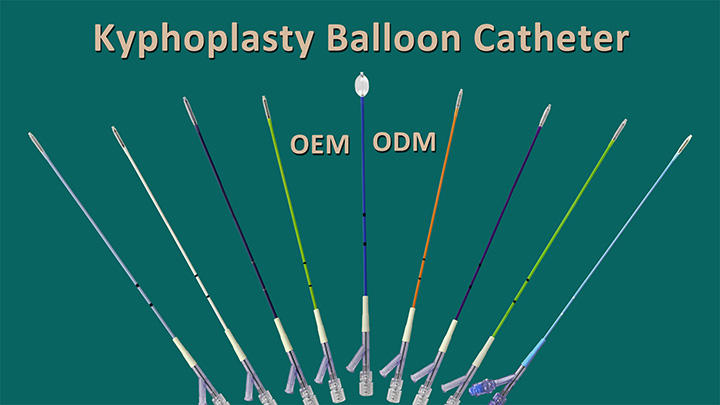
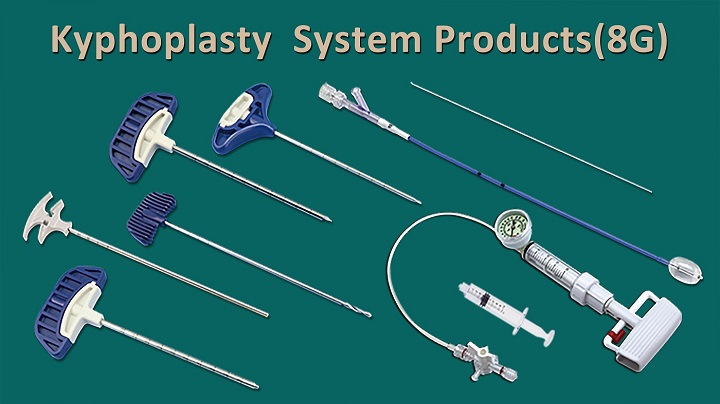
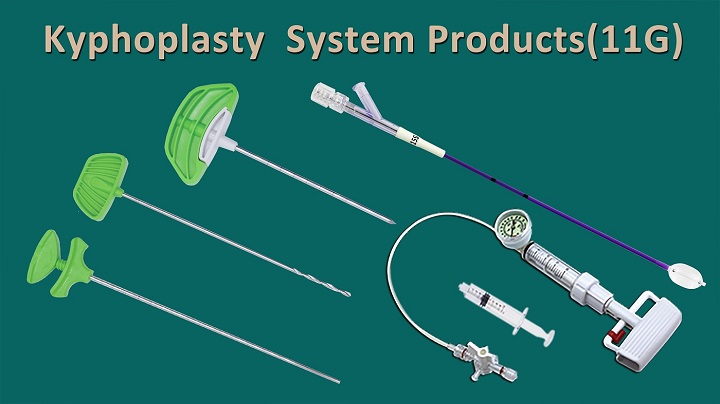





 CopyRight © 2024 - Jiangsu Changmei Medtech Co., Ltd.All rights reserved
CopyRight © 2024 - Jiangsu Changmei Medtech Co., Ltd.All rights reserved
